Cathodic Protection of Iron and Steel
Recent applications to heritage buildings
David Farrell, Kevin Davies and Iain McCaig
 |
|
 |
|
| The Whitchurch almshouses before repairs (top), showing spalling stonework along the base of the front wall caused by the rusting metal cramps, and (below) a schematic diagram showing the SACP system used to protect the cramps |
Metal dowels and cramps were often built into traditional masonry structures to secure stones which might otherwise be prone to movement or displacement, for example, copings, parapets and cornices. They were also widely used in ordinary ashlar walls, which usually consisted of a relatively thin facing of finely dressed and narrow jointed stonework, with a core or backing of rubble or brick. Cramps would be incorporated to tie the facing back to the core. Dowels and cramps were also embedded in the facing itself to help maintain its structural integrity.
In 18th and 19th century buildings, dowels and cramps were usually made from wrought iron which is susceptible to corrosion if exposed to air and moisture. In ashlar masonry of this period it is common to find vertical joints not filled with mortar to their full depth. When the shallow bead of mortar at the surface decays or cracks, rainwater is able to penetrate freely. The narrowness of the joints makes effective repointing very difficult, so water penetration continues, causing the embedded cramps to rust. The expanding rust eventually exerts such pressure on the stone that the stone cracks or spalls. The conventional remedy involves major surgery to remove the cramps, replace them with non‑corroding phosphor bronze or stainless steel and then repair the damaged stonework.
Some 19th and 20th century masonry‑clad buildings incorporate steel frames which are also liable to corrosion. Again, conventional treatments can be highly invasive involving large‑scale opening up to expose and treat the affected components. Cathodic protection offers an alternative approach to the treatment of rusting iron and steelwork buried in masonry and stone.
CATHODIC PROTECTION TECHNIQUES
Cathodic protection (CP) encompasses a range of techniques used to suppress corrosion of metal structures and components. CP is not a new process: in 1824 Sir Humphrey Davy presented a series of papers to the Royal Society describing how CP could be used to prevent the corrosion of copper sheathing in the wooden hulls of British naval vessels. Since then it has been applied in many areas, including marine applications and for the preservation of buried underground structures such as oil pipelines and tanks. CP technology has, over the past 20 years, been applied to reinforced concrete to protect steel reinforcements from corrosion and more recently, it has also been applied to iron and steel embedded in brick, masonry and stone in heritage buildings.
CP systems work on the principle that corrosion is an electrochemical reaction in which one part of a piece of iron or steel acts as an anode while adjacent metal acts as a cathode. At the anode corrosion occurs as iron gives up electrons and forms soluble iron ions. At the cathode the electrons released by the corrosion process combine with water and oxygen to form hydroxide ions. In CP systems the metal to be protected is forced to act as the cathode, as on this side of the reaction the surface of the metal is unaffected by the reaction, preventing further corrosion. When used to protect structural iron and steel this is achieved by applying small DC electric currents, via the building material. This supplies a constant stream of electrons to satisfy the cathodic reaction. The anodic (corrosive) reaction then becomes suppressed. There are two methods of achieving this, either sacrificial anode cathodic protection (SACP) or impressed current cathodic protection (ICCP).
SACP systems use sacrificial anodes (zinc, aluminium or magnesium) which are placed in close proximity to the corroding metalwork and electrically connected to it. As the sacrificial anode corrodes, it generates a current that passes through the building material to provide protection to the embedded metalwork. The current is ionically conducted by means of pore water contained within the building material. These systems are capable of protecting small metal components such as embedded iron cramps or restraints set into walls, floors or roofs of a building.
ICCP systems use transformer rectifiers, normally mains powered, to provide the DC current to the iron or steel being protected. These systems use corrosion resistant anodes, fixed close to the metalwork, to provide part of the current pathway. ICCP systems are more complex than the SACP systems, but are suitable for providing CP to much larger areas of embedded steel such as I-beams, supports and columns and where the stone or masonry has inherently higher electrical resistance.
WHITCHURCH ALMSHOUSES – A SACRIFICIAL ANODE CP APPLICATION
In 1999, an SACP system was installed to protect rusting iron cramps in the stone facade of four Grade II listed almshouses in Whitchurch, Shropshire. This was the first known application of its kind in the UK. The stones formed an interlocked frontage with iron cramps fitted between adjacent blocks. Water ingress beneath the eaves and below window sills had permeated into stonework joints and had allowed the iron cramps to corrode, especially in the vertical joints around windows and doors. The expanding corrosion product had introduced internal stresses into the stonework which had resulted in cracking and spalling of some of the stones.
 |
| A corroding cramp causing masonry to fracture at the Grade II listed almshouses |
Conservation policy (see PPS5 for example) favours the ‘minimum intervention’ principle, whereby any repairs should preserve the historically important features of the architecture. For this structure, a novel repair technique using SACP was adopted. Damaged stones located on the outer edges of the facade were replaced with new stones fitted with stainless steel cramps. For the remaining, as yet undamaged stones, an SACP system was installed to control further corrosion of the iron cramps.
To provide the cathodic protection, six magnesium anodes were buried in the pavement in front of the cottages and these were connected directly through to the iron cramps in a ‘ring circuit’ arrangement. Electrical connections were made to the cramps using a ‘keyhole surgery’ technique to minimise damage to the stones; the titanium connection wires were sunk into the mortar joints; and the current from the sacrificial anodes was conducted through the stone facade thus completing the CP’s ‘electrical circuit’. This allowed the cramps to be polarised from an external source and thus protected from further corrosion.
THE WELLINGTON ARCH – AN IMPRESSED CURRENT CP APPLICATION
 |
 |
| The Wellington Arch, London and a detail (below) showing the corrosion of the iron beams supporting the quadriga |
A number of heritage structures within the London area have been fitted with ICCP over the past few years, including an external staircase at Kenwood House, to protect the embedded steel and the Inigo Jones Gateway at Chiswick House, to protect iron cramps.
An ICCP system has recently been installed in the Grade I listed Wellington Arch, Hyde Park Corner. The arch was built in the late 1820s using Portland stone and the roof slab was formed from concrete, supported by steel I-beams. The arch has recently undergone extensive renovation, including the repair of the steel and concrete structure which supports the bronze sculpture of the quadriga. During the inspection it was discovered that some of the key steel I-beams in the roof had suffered significant corrosion. This was partly due to rainwater penetrating the roof structure. The worst corrosion, however, was where the beams had been in direct contact with the Portland stone.
Portland stone, having a neutral pH, offers no corrosion protection for steel. This may be compared to concrete, which is alkaline and, when in intimate contact with steel, helps to passivate the surface, inhibiting further corrosion. In fact, as a sedimentary rock formed in a marine environment, Portland stone may contain significant concentrations of chlorides or sulphates (salts) which, in the presence of moisture, can accelerate the corrosion process.
Corrosion of the arch’s I-beams had several consequences.
- The corrosion products physically occupied a much greater volume than the original steel and they began to push against adjacent surfaces. The corroding I-beams had been physically displaced upwards by 20mm in places, due to the formation of corrosion products underneath the beams and this had resulted in cracking of some of the stonework. This process is known as 'corrosion jacking' or 'rust jacking'.
- The corrosion products had caused delamination of some areas of concrete on the roof slab allowing further corrosion of the now unprotected steel.
- Substantial thinning of the steel I-beams caused concern about the future integrity of the whole support structure if significant corrosion continued.
THE APPROACH TO RENOVATION
English Heritage did not wish to replace the beams as this would have been both expensive and disruptive, possibly requiring lifting the quadriga. Besides, conservation is about retaining as much as possible of the original structure and conserving its current condition. Ultrasonic thickness readings of the steel beams showed that their remaining thickness was sufficient to support the roof slab and quadriga, provided that ongoing corrosion could be controlled.
INSTALLATION OF THE ICCP SYSTEM
To provide efficient long‑term CP performance, titanium expanded mesh ribbon anodes, with a mixed metal oxide (MMO) coating, were cast into the top surface of the concrete slab above the steel frame structure. This ribbon anode system provides maximum anode surface area for efficient transfer of the electrical current through the structure’s concrete sections.
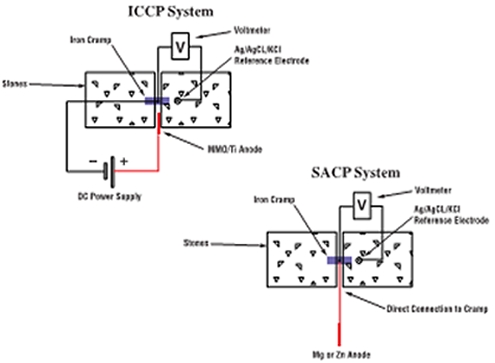 |
| Schematic diagrams of ICCP and SACP systems for corroding iron embedded in stone |
Small, discreet MMO coated titanium rod anodes were used to protect the steel beams where they were laid over the Portland stone. These anodes were embedded into 12mm diameter, 300mm deep holes at centres of 300mm in the mortar pointing of a brick course under the stone, using a conductive backfill to provide electrical continuity. These discreet anodes provide current into the depth of a wall to protect embedded steelwork.
System wiring from the beams and anodes was installed within the internal roof space back to an instrumentation cabinet. The cabinet houses the system electronics and computer for system control and monitoring.
BENEFITS FROM USING THE CP APPROACH
One of CP’s principal advantages in the protection of embedded metalwork is that it provides corrosion protection without changing the immediate physical environment: there may still be now, or in the future, damp concrete or stone adjacent to the metal which would previously have allowed corrosion to continue. Cathodic protection provides the electrochemical conditions to control this corrosion process.
The implication of this is that there is no need to gain full access
to the structure by removing the surrounding material and the structure
can remain largely intact. All that is required is to install the necessary
cables and anodes that form part of the CP system. Usually, these can be
installed in such a way as to have little or no impact on the structure’s
visual appearance. In the case of the Whitchurch almshouses, this
avoided dismantling of the stone facade and removal of the expanded
corroded cramps, a project with uncertain consequences. On the
Wellington Arch, major structural upheaval was avoided by adopting a
CP approach.
~~~
Recommended Reading
- J Morgan, Cathodic Protection (second edition), National Association of Corrosion Engineers, ISBN 0‑915567‑28‑8, 1993
- K Blackney and B Martin, The Application of Cathodic Protection to Historic Buildings, English Heritage Research Transactions, ISBN 1 873936 62 1, Vol 1, April 1998



