Atmospheric Pollution, Climate Change and Historic Buildings
Rob Inkpen
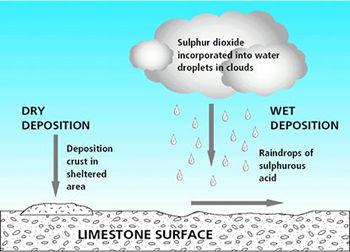 |
| Figure 1: Depositional routes to material surface. Dry deposition on a sheltered surface forms a deposition crust. It can also occur on a surface that is exposed to rainfall and run-off, but here the products of reaction are removed once rainfall and run-off reaches the surface and move across it and into the material. In the case of limestone both wet and dry deposition produce the same alteration; limestone is converted to calcium sulphate (gypsum). The reaction through dry deposition (direct gas to solid reaction) is about five times slower in producing this decay product than the reaction via wet deposition (reaction in solution). |
 |
| Cavernous weathering in sandstone in a wall in Durham. Small particles at the base of each cavern are eroded small flakes of stone mixed with salts. Repointing has isolated each block and focused weathering processes to produce these forms. |
John Evelyn noted that the noxious air of London was causing the discolouration and degradation of buildings as early as the 1660s. Throughout the late 18th and 19th century, painters and writers used the smog and squalid conditions of urban areas to dramatic effect, providing an evocative image of the deleterious impact of urban atmospheres. More recently, there have been attempts to understand the nature of atmospheric pollution and its impacts on buildings in a more systematic and scientific manner.
POLLUTANTS
‘Atmospheric
pollution’ usually refers to the element of the atmosphere which has been altered
by human activities. Changes in natural and polluted atmospheres can be highly
variable both across space and through time. In coastal environments, for example,
there is naturally more salt in the atmosphere than in inland environments. Salting
activities in cold spells, however, create artificially elevated levels of salt
in inland locations.
Some of the major pollutants that effect stonework are carbon dioxide, sulphur dioxide, nitrogen oxides and particulates such as smoke. Carbon dioxide is probably most familiar as a ‘greenhouse gas’, contributing to global warming, but it also combines with water in the atmosphere to produce carbonic acid. This means that natural rainfall is a weak solution of carbonic acid with a pH of about 5.6 (pH 7 being neutral and pH 0 being the most acidic). Even if carbon dioxide levels rise by as much as the most pessimistic predictions, the increase in rainfall acidity this gas will cause is relatively minor. It is the reactions of other pollutant gases, such as sulphur dioxide and nitrogen oxides, that produce the more acidic rainfall. Sulphur dioxide reacts with water in the atmosphere to produce sulphurous acid (H2SO3). This is what is popularly referred to as ‘acid rain’. Acidities of rainfall can commonly be reduced to pH 4.5 or, in the extreme, to pH 3.5 by this pollutant. Nitrogen oxides can produce a similar reduction in pH by the formation of nitric acid, but these oxides have tended to be harder to detect, especially in terms of their impact on stonework.
The above gases are produced by industrial, commercial and residential activities in urban, and increasingly, in rural areas. Carbon dioxide, as has been well documented, is a by-product of much industrial activity and is almost uniform in its distribution around the UK. Sulphur dioxide was a major by-product of the electricity production and domestic heating using coal. Up to the 1950s and the great London smogs, domestic use of coal for heating produced a very concentrated and confined blanket of sulphur dioxide and smoke over major urban areas. One of the effects of this was sulphation, a chemical reaction between the masonry surface and the atmospheric sulphur dioxide, evident on limestone (calcium carbonate) as a black crust of dirt, sulphates and stone which, in the worst cases, cause the surface of the stone to peel away. Increasingly stringent regulations, such as the Clean Air Acts of the 1950s, have greatly reduced the amount of sulphur dioxide and smoke in the urban atmospheres of the UK. The effects of a long history of high sulphur dioxide and smoke levels are, however, less easy to remove from the stonework of the UK.
Nitrogen oxides, like sulphur dioxide, are produced by exhaust fumes and include a family of pollutants, including ozone, resulting from the reaction of nitrogen oxides in urban atmospheres.
There can be a great deal of variation in the amount of pollution found in any particular part of an urban area depending on the local industries, other pollutant sources, topography and climate. This means that the contemporary concentration of pollutants can vary greatly over an urban area. Likewise, transportation of pollution beyond the area of its production can increase pollution levels in seemingly clean areas. In the UK this has meant that rural areas do not represent a pristine environment and cannot be used to gauge the natural, background level of pollution.
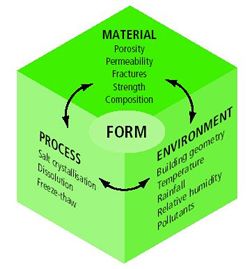 |
| Figure 2: Example of factors influencing the interactions between pollutants and materials. |
CLIMATE
CHANGE
Pollutants
such as carbon dioxide are also having an impact on the general climate. Although
a 2-5°C rise in temperature and a rise in sea-level are predicted over the next
50 years, these general figures hide a great deal of regional variation in climate
change. It is likely that southern Britain will experience a more Mediterranean-like
climate, with increasing periods of drought and dry weather, whilst the distribution
of rainfall is likely to become more seasonal. Similarly, the frequency of ‘extreme’
climatic events, be they droughts or storms, is likely to increase. Such changes
are likely to affect building degradation, but in ways currently difficult to
predict.
The duration and intensity of driving rain, for example, is likely to increase as extreme wind speeds increase along with storminess. As well as increasing the penetration of rainwater into a building, there may also be an increase in the amount and location of runoff generated during rain events. Increased driving rain may mean that previously sheltered areas experience runoff for the first time, removing any sulphation crusts. Likewise, increased penetration of moisture may activate salts passively accumulated and stored in the building material. Once activated, the salts cause harm by migrating, in solution, to the point where the moisture evaporates. Here they accumulate and crystallise. If the crystallisation takes place within the pores of the masonry, crystal growth can exert enormous pressure on the walls of the pores, causing the stone to crumble. Thus the ‘memory effect’ of salts acquired either in the formation of the stone or subsequently, can produce accelerated rates of weathering without any change, or even with a decrease, in the pollutants in the atmosphere. Contemporary weathering is thus dependent on the constituents of the building materials and the past weathering history of the material, as well as on current pollution.
POLLUTANTS
AND MATERIALS
Materials
and pollutants interact in a complicated manner. Dry deposition, the direct deposition
of gaseous pollutants onto a surface, varies with factors such as wind speed,
building orientation and relative humidity. For sulphur dioxide, for example,
the reaction of the gas with limestone is enhanced if relative humidity is above
80 per cent. Wet deposition, the delivery of gaseous pollutants to a surface via
their incorporation into water, varies with factors such as the geometry of the
surface. Where a surface is exposed to rainfall and runoff, then wet deposition
can occur and chemical reactions such as dissolution can take place. The interaction
between pollutants and materials highlights the importance of specific physical
properties for the vulnerability of materials. A matrix of calcium carbonate or
a calcium carbonate rock is highly susceptible to reactions with acid solutions.
Porous materials, whatever their chemical composition, are likely to be vulnerable
to degradation by acid solutions, as their large pore volume provides a large
surface area for chemical reactions. Similarly porous or fractured material will
also be susceptible to the actions of salt, as salts can penetrate into confined
spaces where their expansion can exert great stress upon the material. Lastly,
the presence and movement of moisture within a material, facilitated by high porosity,
can enhance and alter the concentration of weathering agents and aid their damaging
activity.
Glass can undergo chemical alteration in polluted environments, although this may not be visually obvious at first on modern glass. Sodium can be leached out of the surface layers of modern glass in atmospheres rich in sulphur and carbon dioxide. Medieval glass can be leached of its potassium and calcium content, weakening the structure and durability.
Metals can also be chemically altered within a polluted atmosphere. The degree of alteration depends to a great extent on the presence of moisture (and how long the metal remains wet), the concentration of pollutants present and the supply of oxygen to the reacting surface. If the supply of oxygen is cut off by the formation of a layer of weathering products, the reactions can slow down dramatically. Oxidised layers may actually form a protective barrier to the further alteration of the metal surface. If these layers become wetted, however, there may be a rapid electro-chemical reaction, enhancing alteration.
FORMS
OF WEATHERING
Three
sets of interrelated factors determine how materials interact with atmospheric
pollutants and the form of decay; material, environment and process. Unfortunately,
this complex set of relationships means that it is often difficult to establish
a clear and distinct one-to-one relationship between pollutants and decay forms.
It may be that the same pollutant can produce different decay forms depending
on the specific circumstances of interactions, or it may be that the same form
can be produced by a set of different pollutants. It is also important to note
that some reactions will only occur if the level of pollutants or an environmental
parameter crosses a critical threshold. Salt weathering, for example, may not
occur unless pore spaces are filled and stress can be exerted on the pore walls.
Classification schemes for the various forms of decay are varied and often assume that forms are produced by specific processes. Visually, a simple distinction can be made between areas of a building washed and unwashed by rainfall and runoff. Washed areas can be dissolved by rainfall and runoff, and localised dissolution can be produced where small gullies or runnels form. Rain-washed surfaces often show the effects of dissolution by having a roughened surface, in some cases such as marble, a sugary feel to the surface. Sheltered surfaces, particularly where calcium carbonate is present, can produce sulphation crusts, blackened by the incorporation of carbon particles into the crust. The location and extent of these forms depends upon the configuration of the building and how heavy the rain is and how often it falls. As climate alters, the location of these sheltered areas could change.
In addition to simple dissolution surface and crusts, a further series of weathering forms are those produced by the action of salt within the material. Efflorescence of crystals on a surface may indicate the presence of salts, such as sodium chloride or calcium sulphate, but need not indicate that these salts are causing damage to the material. If salts move rapidly through a material to the surface they may not exert sufficient stress to cause damage. Salt crystallisation within constrained volumes, such as pores, can exert great stresses within the material and result in its breakdown. The form of this breakdown can vary and produce forms such as flaking, blisters and honeycomb weathering.
Weathering forms, as noted above, need not be dependent upon current pollutants for their development. Once a blister is produced on a surface, for example, that surface is weakened and salt migration enhanced within the blister. The weathering form may follow a sequence of development independent of any change in atmospheric pollution levels. Identifying these weathering pathways is still very much a case of experience rather than systematic analysis.
Recommended Reading
-
The effects of acid deposition on buildings and building materials in the United Kingdom, Building Effects Review Group Report, HMSO, London, 1989
-
P Brimblecombe (ed), The effects of air pollution on the built environment, Imperial College Press, London, 2004
-
R Prikryl and HA Viles (eds), Understanding and managing of stone decay, The Karolinum Press, Prague, 2002
-
RJ Schaffer, The weathering of natural building stones, 1932, DSIR Building Research Special Report 18, 1985
-
BJ Smith and PA Warke, Stone decay: Its causes and controls, Donhead, Shaftesbury, 2004
Websites
www2.rgu.ac.uk/schools/mcrg/mcrghome.htm. Masonry Conservation Research Group: Robert Gordon University, Aberdeen (A good site for links to a wide range of organisations and groups concerned with conservation of building materials).
SWAPNET is an informal network of academics and other parties interested in stone decay and conservation. The group acts as a forum for discussing ideas and techniques for monitoring and understanding decay. Recent meetings have been held in Prague, Oxford and Belfast from which a series of publications has been produced. If you want further information on this group please contact Rob Inkpen (robert.inkpen@port.ac.uk).



