Painting Historic Ironwork
Keith Blackney
 |
||
 |
||
| This weather vane, at St Mary's Church, Hendon, was repaired by Architectural Metalwork Conservation, grit blasted and then zinc sprayed before being painted with an epoxy sealer/primer and build coats, and finished with an acrylic urethane. This high level of intervention and the use of a difficult-to-reverse system was justified in this case as all the original iron surfaces had been lost to severe corrosion and regular maintenance will be impossible at such a height. Gold leafing was carried out by Howell & Bellion. |
Lots of names are thrown up when considering the most appropriate coating for historic ironwork: oil paint, lead paint, alkyds, micacious iron oxide, epoxies, two-pack, zinc phosphate, zinc-rich, iron oxide and polyurethane, to name but a few. So it is little wonder when faced with this barrage of choices that specifiers often retreat to using a standard specification without considering the particular requirements of the historic fabric. Over-specification may be harmful to the historic fabric: inappropriate treatment may lead to premature failure of the coating. The success of a coating specification for ironwork can only be assessed once it has begun to fail, and the optimism always felt when viewing new paintwork can be a false portent of its true effectiveness.
The choice of coating will be influenced by many different criteria, including historic appropriateness or authenticity and decorative effect, but perhaps the most important consideration is its effectiveness in protecting iron from corrosion.
Water and oxygen combined in contact with iron will initiate the electrochemical process that is corrosion. This results in the metal being eaten away as electrons are freed from iron atoms, characterised by the formation of hydrated iron oxide (rust). The rate of corrosion may be governed by a number of factors: the chemical and physical condition of the metal; contact with metals of differing electrical potential to release electrons; and environmental factors, including concentrations of moisture and oxygen, air flows, chemical pollutants and temperature.
THE COMPOSITION OF PAINT AND OTHER WET-APPLIED COATINGS
Paints are liquids which dry through solvent evaporation usually in conjunction with oxidation or other chemical reactions. Other coatings found on historic metal which behave in this way include varnishes, bitumen and tar.
Paints and varnishes are generally made up from solvents, binders and pigments. Solvents, for example white spirit in modern oil paints, dissolve the binder (resins and oils) into a liquid. Initial drying takes place as the solvent evaporates leaving the binder, which then further hardens by polymerisation, a chemical reaction in which smaller molecules link together to form longer molecular chains. This is brought about by either the chemical reaction of two constituents which are mixed together just prior to application, or by oxidation (mainly contact with air) which leads to the formation of a hard film across the surface of the coating. This gives rise to the terms two-pack or film-forming (single-pack) coatings.
Binders include natural oils, alkyd oils, resins, vinyl and chlorinated rubber. The choice of binder has significant influence over the character and performance of the coating. For example, surface tolerant coatings, suitable for application over less scrupulously clean surfaces, will contain binders with good wetting properties.
Pigments are the small solid particles that provide corrosion protection or inhibition in primers as well as opacity and colour in all coats. Primers are usually named according to their pigments, the most often encountered examples being aluminium, iron oxide, red lead, zinc phosphate, and 'zinc rich', which contains metallic zinc dust. Protective pigments are also sometimes found in other coats, the most well known of these being micacious iron oxide, added to intermediate/build coats to improve the dry film's moisture barrier properties. Pigments can be carried in a range of binders. For instance, zinc phosphate can be found in alkyd, chlorinated rubber and epoxy resin binder paints.
Paints are usually applied in systems which usually have two to four coats and sometimes more, (preferably in distinct colours or shades) comprising: primers, build coats, undercoats and finishes. A primer is designed to provide a key for the subsequent coat and usually also provides corrosion protection and inhibition. The build coat is also likely to contain additives to protect the metal, but this layer is also designed to add film thickness. Depending on the colour and texture of the build coats, the undercoat is often omitted as its main purpose is to provide a decorative base for the finishing coat.
TRADITIONAL COATINGS
The most common type of original coating system found on historic ironwork is traditional oil paint made up of a turpentine solvent, linseed oil binder, white lead (lead carbonates and sulphates) pigment, and other pigments and additives, principally dryers. These were usually applied over a corrosion-inhibiting primer which, in the 17th and early 18th century, typically contained red ochre ('Spanish brown') and a small proportion of white lead. Red lead, which can be distinguished from red ochre by its lighter colour, was sometimes also used in primers before the mid 18th century, and it became the most common pigment in linseed oil primers in the late 18th and 19th centuries. Other traditional coatings include pitch, coal tars and bitumen, primarily used on engineering structures such as bridges, piers and aqueducts.
Traditional coatings can have remarkable durability, with recent discoveries on exterior ironwork dating to the Jacobean period. Traditional oil-based paints tend to dry slowly and often need to be applied in several thin coats. This constraint, along with issues of lead content, the constant demand for labour-saving materials and the development of 'high performance, low maintenance' coatings for industry, has led to the almost complete disappearance of traditional oil paints for coating ironwork. However, following the trend towards the use of traditional materials in general, there is a major re-evaluation of traditional coatings. In respect of ironwork, the interest stems from the work of paint analysts researching authentic colour schemes as alternatives to the ubiquitous black. There are also strong technical reasons for considering traditional coatings. Firstly, they are likely to be the most compatible materials for over-coating original materials. Furthermore, their tolerance to application over iron with forge and foundry finishes and less than perfectly cleaned rusted surfaces, coincides with resolving the conundrum of minimum intervention versus the vigorous surface preparation methods often specified for modern paints.
MODERN PAINTS
The most common singular replacement for traditional oil paint is paint with alkyd oil binders. These single-pack materials offer a higher build (film thickness) from one application and faster drying than traditional oils. Premium products may contain additives: silicone, for example, is added to gloss finishes to improve long-term performance against weathering.
As a general rule, alkyd oil coatings and traditional oil paints fall into the category of coatings which have the shortest service life, requiring the most frequent maintenance. Yet when compared to the more industry-oriented higher performance coatings, alkyd oils do have the advantages of being both widely available from ordinary paint stockists and relatively easy to use.
Other single-pack coatings offering a longer service life, particularly in adverse environments, include solvent drying vinyls and chlorinated rubbers, and moisture-cured urethanes in which a rapid chemical cure is brought about by airborne moisture. Meanwhile, two-pack epoxies, usually decoratively over-coated by two-pack polyurethane coatings, are considered to offer the longest life between major maintenance.
WAX AND OIL TREATMENT
In addition to paints there are a number of other coatings which are sometimes used on ironwork, including oils and waxes.
The principles of minimum intervention and reversibility are best served by wax and oil treatments. Prior preparation of the metal can be limited to washing down to remove dirt, and the coatings are usually simple to remove. However, they have the disadvantage of requiring very regular maintenance and, while sometimes used on interior ironwork, they are usually only applied externally to stainless steels and nonferrous metals such as bronze.
GALVANISING AND SPRAYED METAL FINISHES
At the other extreme, there are a number of long lasting treatments which cannot be applied or removed without considerable intervention, inevitably resulting in the removal of the original substrates. Hot dip galvanising, first developed 160 years ago, is resistant to abrasion damage and provides cathodic protection due to its sacrificial corrosion at small breaks in the cover. The coating is both the most effective method of protecting ironwork against corrosion and the most difficult coating to remove. It is ideal for new functional steelwork but has no place as a replacement for paints on historic pieces, as the process leaves a layer of zinc alloy chemically bonded with the iron.
 |
|
| Railings at Countess of Huntingdon's Chapel, Bath. Samples taken from the earliest railings (below left) suggest that when the chapel was originally built in 1765 they were painted grey. (Lisa Oestreicher Architectural Paint Analysis) | |
 |
 |
Applied over grit blasted surfaces, thermal sprayed ('metal sprayed') zinc and aluminium coatings are sometimes used in restoration work as well as in new work as they offer a physically tough barrier and cathodic protection. They are less likely than galvanising to mask fine decorative detail and their slightly roughened surface finish offers a key to paints. However, the spray method of application limits the operator's ability to reach hidden detail and penetrate joints. This disadvantage is sometimes reduced by flooding the unreachable areas with zinc rich paints, but the process remains most beneficial on components which can be fully exposed for treatment.
Galvanised ironwork is ready for service, while thermal sprayed coatings are usually further treated with a low viscosity sealer. Both processes can be over-coated for increased protection or more usually for decoration. Subsequent primers must be suitable for non-ferrous metals; mordant solutions (known as 'T wash') and etch primers are often used to prepare the surface. The full range of wet and powder coatings is used for overcoating, yet it should be noted that oil binder paints can prematurely fail due to saponification (alkaline attack).
INTUMESCENT PAINTS
Where fire protection is required for structural ironwork, it may be possible to use an intumescent paint. This will swell when exposed to heat, providing insulation which will delay the time before the iron buckles or fractures. Intumescent paints will change the appearance of the structure due to the high film thickness required and they are available only in a limited range of colours.
SURFACE PREPARATION AND PAINTING
 |
||
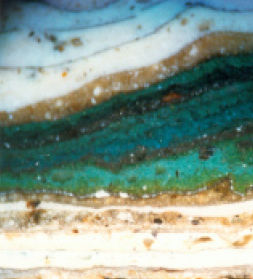
| Micrograph showing a section through a paint layer revealing a total of 37 schemes, 35 of which are traditional lead paints. Orange fragments of the priming layer can be seen at the bottom of the sample followed by two thin layers of a grey scheme. Subsequent schemes include cream and stone, sky blue and many brightly coloured layers. Only the most recent scheme is black. (Lisa Oestreicher Architectural Paint Analysis) |
There are several publications designed to help in the selection of coating systems and which give advice on their application. The obsolescent British Standard BS 5493:1977 and its successor BS EN ISO 12944 Parts 1-8: 1998 are particularly useful. They set out a range of systems from surface preparation through to finish coat and project their performance in a variety of environments.
CLEANING METALWORK
Before carrying out major repairs to ironwork it is current usual practice to remove all corrosion and existing coatings. Although this approach may not be appropriate, particularly where the paint coating is itself historic, it has a number of clear advantages, including facilitating repairs and removing materials which are potentially hazardous to health, while also allowing for archaeological investigation of the underlying metalwork and revealing hidden defects.
Thorough stripping may also improve the effectiveness of the new coatings and provide the desired aesthetic rejuvenation. However, some original surface finishes (oxides for example) as well as some corrosion products can themselves make a stable crust to reduce the speed of attack on the underlying metal. The need for cleaning must be weighed against the risk of accelerated decay and loss of historic material rather than be carried out as a matter of course.
The importance of the existing coating should always be considered before removing it, whatever the material or component. Historic layers of paint on iron and other architectural elements offer an insight into the appearance of a building (internally and externally) and its surroundings at different points in its history. Paint layers may also provide invaluable information on the age of a component, and the date alterations were made, and will also provide an insight into past coating technology (see The Archaeology of Decoration by Lisa Oestreicher in The Building Conservation Directory, 2001).
If the surface of important historic metalwork is to be stripped of coatings, a specialist should first be brought in to take samples, so that the historic/archaeological information can be recorded.
 |
 |
||
 |
 |
||
| In addition to cast, wrought iron and steel gates and railings, other iron features found on churches and in churchyards include door furniture, rainwater goods, ridge crestings and weather vanes, light fittings and many other features | |||
Severe corrosion on historic ironwork is typically localised around difficult to paint areas which are liable to retain moisture, for example where leaves spring from bars. Adjacent plain bars will frequently remain in perfect condition beneath a lifetime's accumulation of paints. A range of targeted cleaning methods may be required in order to prevent further deterioration in the difficult to paint areas, while avoiding unnecessary disturbance of sound material. Aesthetic considerations aside, it may only be necessary to remove loose paint and corrosion in addition to any grease and dirt which will compromise the all important adhesion and unbroken coverage of the new coating.
Appropriate surface preparation is vital if the new coating is to reach its full service potential. The main methods of removing corrosion and old coatings include: hand and power tool cleaning using scrapers, wire brushes and chipping tools such as needle guns; chemical stripping; flame cleaning; air abrasive methods commonly described as shot blasting and grit blasting; high pressure water blasting. All these methods can damage ironwork and their success depends on the skill, experience and judgement of those carrying out the process. These methods are discussed in detail in The Building Conservation Directory 2002.
Whatever method of cleaning ironwork is chosen it is important to bear in mind that the removed material may be highly toxic. In particular, historic paint may contain leads and other heavy metals, with implications for both personal health and the environment. When planning the work, consideration must be given to the control and disposal of all waste material.
APPLICATION
 |
||
| The use of lead carbonate or lead sulphate in paint is banned in the UK except for 'the restoration and maintenance of an historic building, or fine decorative work of art, where it is required to maintain historic textures and finishes' (see the Environmental Protection (Controls on Injurious Substances) Regulations 1992: Statutory Instrument No 31). Those containing red lead continue to be available unlicensed from specialist manufacturers. |
Applying the coatings within suitable temperature and atmospheric parameters is crucial to achieve the desired protection and decoration. Coatings manufacturers provide advice on these and other technical aspects together with product data and health and safety sheets that should be studied before final commitment to a system. Wet paints can be applied by brush, roller or a number of different spray methods. Again reference should be made to the manufacturer's literature to ensure the most suitable techniques are used for a particular product, and to be aware of any specific health and safety issues which may arise, as well as any environmental concerns. Roller and spray methods have clear advantages of speed, but skilled brush application is the method most likely to reproduce historic finishes. Brush application also encourages thorough application to joints, crevices and difficult to reach areas on highly decorated pieces. Whatever the method of application it is vital to ensure that the coating is within specification thickness throughout, and that it is free of any breaks which might expose the iron to the agents of decay.
Ideally, during the metalwork repairs, areas liable to trap moisture should be modified to allow drainage. Where this is not possible or ethically acceptable, particular attention should be given to ensure good film coverage. This can be achieved by flooding with paint, or applying paint-compatible fillers. Red lead putty and compounds primarily consisting of pitch are encountered on existing ironwork and have proven to be effective. Meanwhile, acetic acid free silicones and polysulphide mastics are used in conjunction with modern high performance coatings. Imperfections in the metal's surface, in particular blow holes (casting flaws) in cast iron may be exposed by thorough cleaning. These also need to be filled. Traditional fillers are generally red and white lead putties, and 'Beaumont's egg', a mixture of iron filings, grease and ammonium chloride. Modern fillers are usually based on epoxy or polyester resins.
Finally to maintenance: unfortunately, it is generally the case that this crucial area of preserving fabric rarely receives the financial support and attention lavished on major restorations; themselves often carried out in response to long-term neglect. Little and often should be the mantra of those charged with caring for historic ironwork.



